
1. Pre-freezing step
Usually the first "process" step of the freeze dryer to start the freeze drying cycle. In this process, the product is cooled and frozen, thereby converting the solution in the product from liquid to solid, creating initial conditions for the subsequent sublimation drying. There are two goals to consider for the pre-freezing step, namely the (stage/final) target temperature and the time to reach this target temperature, which can be converted into a pre-freezing rate.
The reason why pre-freezing is so important is not only because it requires supercooling to the critical temperature at which the product changes from liquid to solid, but also because, apart from some uncontrollable factors, the speed and degree of supercooling may also affect the shape of ice crystals, which in turn affects the primary drying and even the quality of the product.
2. Ice crystal morphology
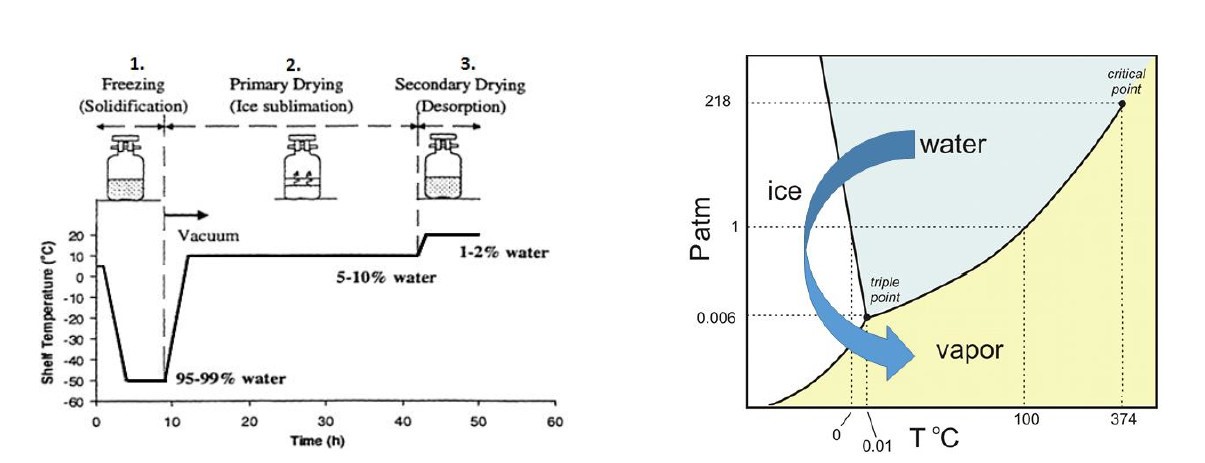
Many "educational" materials will mention that rapid cooling leads to the formation of "small" ice crystals, while slow cooling leads to the formation of "large" ice crystals. The simple understanding is that rapid cooling makes the water supercooled without being prepared, and when it reacts, it has been frozen. Due to time constraints, the ice crystals cannot achieve "great unity", so the ice crystals are small. The size of the ice crystal determines the size of the voids in the dry solid, so small ice crystals create narrow water vapor sublimation channels, increase sublimation resistance, and thus require longer primary drying time to remove crystal water. On the contrary, slow cooling has the opposite effect.
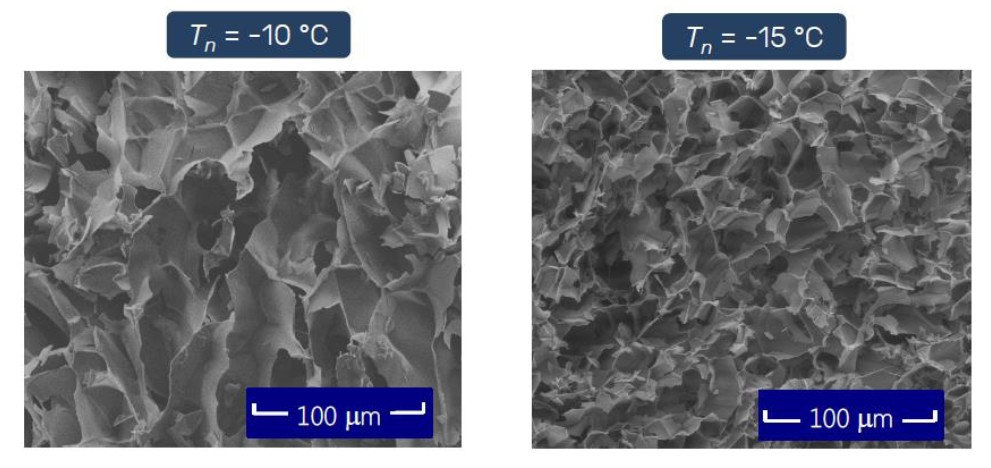
3. Product quality
First, the effect of rate on ice crystal morphology is extended. The size of ice crystals directly affects the size of specific surface area. For example, in protein solutions, freezing will produce an ice-water interface, and proteins will be adsorbed on this interface, thereby destroying their natural folding state. The accompanying loss of secondary and tertiary structures can lead to surface-induced denaturation. However, slow freezing may also increase damage to proteins. For example, as water crystallizes, the possibility of phase separation increases, and slow freezing provides enough time to be exposed to high concentrations of different chemicals, pH changes, and phase separation.
Second, freezing may damage cells for a variety of reasons (such as solution effects, extracellular freezing, and dehydration), but intracellular freezing is the most important reason. The formation of intracellular ice increases the concentration of intracellular electrolytes, which in turn affects the ionic interactions that can participate in stabilizing the natural state of intracellular enzymes. Usually at high cooling rates, when cells cannot maintain osmotic equilibrium with the environment, intracellular ice forms. Intracellular ice crystals may directly cause mechanical damage to the cell ultrastructure, or cells may also be affected by the volume expansion caused by the formation of ice.
4. Influence of the cooling rate of the freeze dryer plate layer
Obviously, the cooling rate mentioned above is based on ideal thermal conditions for the product, and should not be simply attributed to the cooling speed of the silicone oil temperature in the freeze dryer plate layer. For example, the same freeze dryer plate layer cooling rate, facing different products, different filling forms, different filling/loading volumes, and different product initial temperatures, will also bring different cooling effects on the product itself.
But the reality cannot be avoided. If the product is not frozen quickly by offline freezing equipment, then the temperature reduction of the product is more dependent on the temperature change of the freeze dryer plate layer to achieve and control. A friend once asked, many articles use 1°C/minute as a dividing line between fast and slow (whether the definition of this value makes sense is not discussed here), but why the plate layer cooling index in the URS of many freeze dryers is not expressed in rate, but in temperature range and time, for example, "the time from 20℃ to -40℃ should be ≤ 60 minutes, not 1°C/minute". In my opinion, first of all, the indicators of freeze dryers are mostly no-load indicators. When testing their performance, there is no need to perform "linear" slope control, but to check where the performance boundary of the equipment is. The performance of conventional refrigeration systems is affected by many factors and is not a linear performance. The lower the temperature, the slower the rate. Therefore, the performance of no-load equipment is only confirmed to understand its boundary.
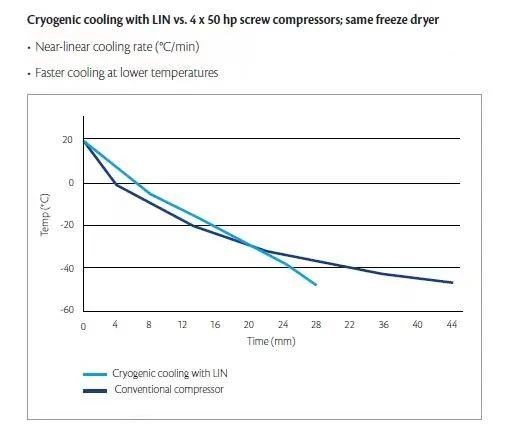
This is because the performance boundary of the equipment is very important. Precisely because its extreme cooling curve is not linear, its worst performance will become the short board of the "barrel", affecting the achievable slope of the cooling curve; in other words, if the freeze dryer plate layer cannot maintain a constant cooling rate within the "required" temperature range in the actual freeze-drying process, it will bring additional unnecessary influencing factors to the product freezing rate.





