
Freeze drying is essentially a process of heat and mass transfer, which is based on the change of the triple point of water. Water is the most abundant substance in the freeze-dried material, so the starting point of our research is water.
1. Three-phase diagram
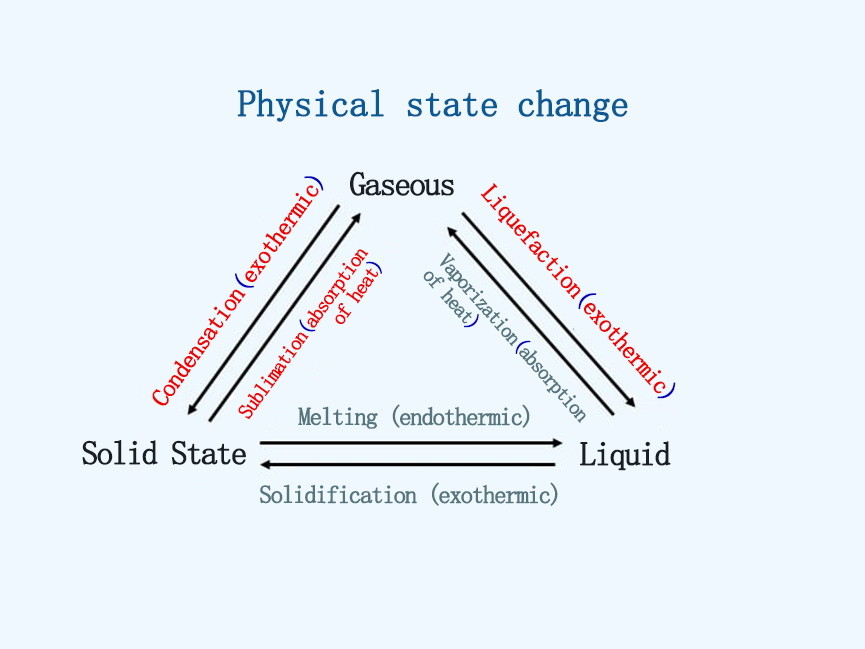
At the triple point, water exists in three states, which correspond to the three states of matter, namely solid phase, liquid phase, and gas phase. The three states can transform into each other. Generally speaking, the process from solid to liquid, liquid to gas, and solid directly to gas is from a state with dense molecular arrangement and strong interaction force to a state with sparse molecular arrangement and small interaction force. This process requires heat from the outside world. The opposite process releases heat to the outside world.
As shown in the figure below: The three states can transform into each other, and the corresponding different processes are sublimation, condensation, liquefaction, vaporization, solidification, and melting.
2. Triple point curve
The evaporation rate of liquid water increases with the increase of temperature, reaching the fastest at 100°C, at which time boiling occurs. As long as it is in an open environment at normal altitude, no matter how high the outside temperature is, the temperature of water will not exceed 100°C. It will continue to boil at this temperature until it is completely converted into water vapor, so 100°C is also called the boiling point of water, that is, the boiling point of water under the corresponding atmospheric pressure is 100°C. And this is an open environment, that is, under standard atmospheric pressure. As the altitude increases, the air pressure decreases, and the boiling point of water will also decrease. If it is a closed environment, water will not be able to escape after it becomes water vapor, which will cause the air pressure in the space to become higher and higher, which will also cause the boiling point of water to change. This principle is used when cooking with a pressure cooker. Since the boiling point changes with the air pressure, if the boiling points under different air pressures are marked, a curve can be obtained, which is the critical curve for the mutual transformation between the liquid phase and the gas phase of water.
In the same way, not only the boiling point is affected by air pressure, but also the melting point (freezing point) and sublimation point (condensation point) are affected by pressure, and similar curves can be drawn. Plotting the boiling point curve, melting point curve, and sublimation point curve on a graph will give you the three-phase diagram of the substance.
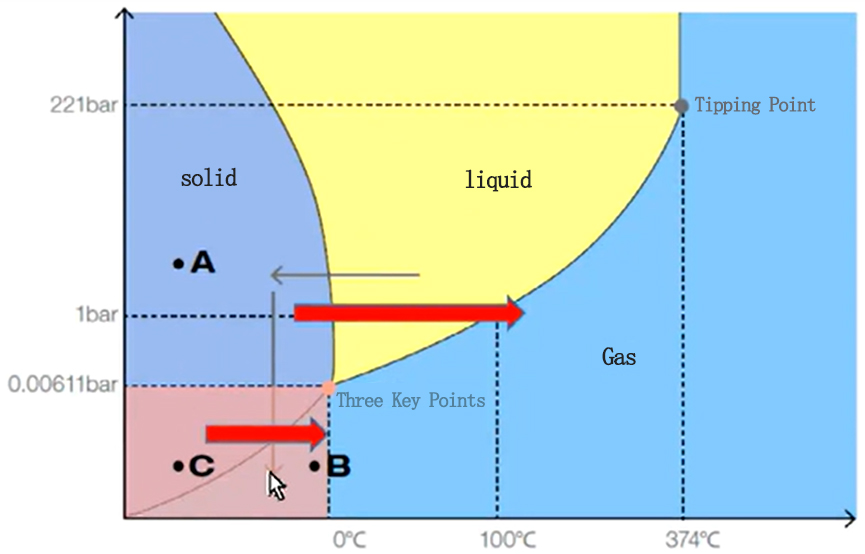
The vertical axis is pressure, and the horizontal axis is temperature. The dark blue part of the figure is solid ice, the yellow part is liquid water, and the light blue part is gaseous water vapor. The curves at their intersection are the solid-liquid phase transition curve of the melting point/freezing point changing with pressure, the liquid-gas phase transition curve of the boiling point changing with pressure, and the solid-gas phase transition curve of the sublimation point/condensation point changing with pressure. The point where the three lines intersect is called the "triple point" of the substance. For water, its triple point is at a temperature = 0.01°C and air pressure = 0.00611mbar. Under this specific environmental condition, not only do the three states of ice, water, and water vapor exist at the same time, but water boiling can also be observed.
At the triple point, the three states of water can be seen at the same time. Freeze-drying can be understood from this triple point diagram.
In order to remove water, the solution is first frozen, and then the water in the frozen solution is sublimated by vacuuming to reduce the air pressure. This approach may seem incredible at first glance, but it is not difficult to understand when combined with the three-phase diagram of water. Place ice cubes in a closed environment at -20°C, remove the air and lower the air pressure until the air pressure is lower than the solid-gas phase transition curve in the three-phase diagram. The ice will then transform into water vapor and dissipate through sublimation. This method of first freezing and then removing water through sublimation is called freeze drying, or "freeze drying" for short. This is the origin principle of freeze drying, or vacuum freeze drying technology.





